David Julius, Ph.D., professor and chair of the Department of Physiology and Morris Herzstein Chair in Molecular Biology and Medicine at UC San Francisco, has won the 2021 Nobel Prize in Physiology or Medicine.
Julius received the prize jointly with Ardem Patapoutian, a UCLA alum and former UCSF postdoctoral fellow, “for their discoveries of receptors for temperature and touch,” according to the Nobel committee in Stockholm, Sweden.
"Congratulations to David Julius and Arden Patapoutian on this richly-deserved honor," University of California President Michael V. Drake, M.D. said. "Their discoveries on pain sensation represent a major breakthrough in how we understand human perception. Insights like these hold immense promise for patients.”
A biochemist and molecular biologist, Julius’s work has focused on how our bodies sense heat, cold and chemical irritants, leading to new insights about the fundamental nature of pain and new targets for pain therapy.
To understand how signals responsible for temperature and pain sensation are transmitted by neural circuits to the brain, Julius and his UCSF laboratory have taken advantage of a variety of noxious substances produced by animals and plants — including toxins from tarantulas and coral snakes; capsaicin, the molecule that produces the “heat” in chili peppers; and the chemicals underlying the pungency of horseradish and wasabi.
Guided by studies of how these natural products and other compounds trigger sensations of heat, cold, and pain, Julius has homed in on a class of proteins called TRP (pronounced “trip”) ion channels as key players in the nervous system’s pain signaling apparatus. One indication of the importance of this work to medicine is the intense interest in TRP channels by the pharmaceutical industry as potential targets for new painkillers.
“David’s work epitomizes the creativity, scientific rigor, and courage needed to pursue the major unsolved mysteries of biology and achieve the surprising discoveries that ultimately lead to crucial advances in human health,” said UCSF Chancellor Sam Hawgood, MBBS. “These are values UCSF is proud to foster on campus, and I want to congratulate David for this well-deserved honor.”
“Congratulations to David for succeeding on the highest scale,” added Talmadge E. King, Jr., M.D., dean of the School of Medicine. “At UCSF, our ability to take excellent care of patients is based on a strong foundation of science, and of researchers like David who have dedicated their lives to discovery. Just as his research opens up new avenues for drug development, his commitment to the education and mentorship of future scientists inspires those around him to push the boundaries of what’s possible."
Study of toxins, and other natural products has led to new targets for pain management
Julius’s career has been driven by an acute awareness of the pressing clinical need for new drugs that could effectively treat pain without the side effects and addictive potential of opioid drugs.
After joining the UCSF faculty in 1989, Julius developed the strategy of using a natural product called capsaicin — the chemical compound responsible for the spicy heat of chili peppers – to track down the protein responsible for the sensation of burning pain. The research led to Julius’s identification and cloning of the specific protein responsible, named TRPV1, in 1997.
Julius’s work revealed that TRPV1, a specialized ion channel located at the outer tips of sensory nerves, responds both to the capsaicin in “hot” peppers and to temperatures greater than 110 degrees Fahrenheit, both of which are transmitted to the brain as a sense of heat. TRPV1 also contributes to the hypersensitivity to heat felt in injured tissue, such as sunburned skin, where mild stimuli can be perceived by the brain as burning hot.
A similar approach enabled Julius and his team to identify and study distinct TRP channels responsible for other types of sensation. Just as capsaicin revealed that TRPV1 allows sensory nerve cells to respond to heat, Julius’ lab used menthol from mint and related compounds to identify a distinct channel called TRPM8 that responds to cold temperatures in 2002. A third TRP channel, TRPA1, responds to the pungent compounds that give wasabi its punch, and is also involved in inflammatory pain.
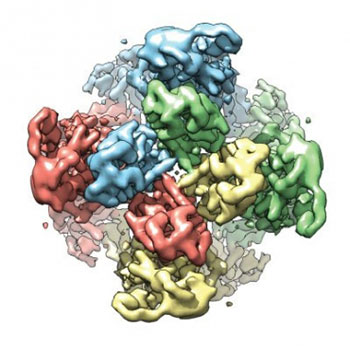
In recent years, Julius has turned his attention to better understanding the structure of TRPV1 and related molecules, in hopes that this information could drive the design of new pain drugs. In a scientific tour de force in 2013, he and UCSF colleague Yifan Cheng, Ph.D., used a technique called cryo-electron microscopy, or cryoEM, to determine the structure of TRPV1 at near-atomic scale. In 2015, Julius and Cheng used cryo-EM to determine the structure of TRPA1, the “wasabi receptor.”
“David’s work is never derivative,” said Allan Basbaum, Ph.D., a frequent scientific collaborator and the chair of UCSF’s Department of Anatomy. “It’s always groundbreaking, seminal.”
Julius has received numerous honors and awards, including the Breakthrough Prize in Life Sciences, Canada Gairdner International Award, the Shaw Prize in Life Science and Medicine, the Kavli Prize in Neuroscience, the Dr. Paul Janssen Award for Biomedical Research, the Passano Award, the Prince of Asturias Award for Technical and Scientific Research, the Scolnick Prize from the McGovern Institute for Brain Research, the Unilever Science Prize, and the Klaus Joachim Zülch Neuroscience Prize. He is a member of the National Academy of Sciences, the National Academy of Medicine, and the American Academy of Arts and Sciences.
About UCSF: The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area.

